The First Cell by Azra Raza (Book Review)

Note: I have a technical accompanying post on my other website which analyses trends in Canadian cancer statistics.
The history of medicine is intimately linked to the study of cancer. Theodor Boveri’s finding that chromosome damage can cause uncontrolled cell proliferation and Peyton Rous’ discovery of a sarcoma virus were critical breakthroughs in early genetics research. After DNA was established as the chemical basis of heredity, remarkable advancements were made in the field of cell biology. The discovery of oncogenes and tumour suppressor genes in the 1970s revealed that cancer is ultimately a genetic disease. Unfortunately these intellectual achievements have not led to effective treatments for most cancers.
No one is more disappointed in the translational failure of cancer research than Dr. Azra Raza, an oncologist and researcher at Columbia, who has written a scathing denunciation of her field in The First Cell. Raza treats patients with the preleukemic condition myelodysplastic syndromes (MDS), as well as its subsequent malignancy acute myeloid leukemia (AML), which develops in around one-third of MDS patients. Being a colleague of Siddhartha Mukherjee, who wrote The Emperor of All Maladies, Azra is following a tough act for writing a cancer book. Yet the field needs more sceptical voices; and Raza certainly provides this.
Each chapter of the book takes it name from one of the author’s patients or someone who died of cancer. Most poignantly, the Harvey chapter takes it title from her husband, a fellow physician who was the director of the Rush Cancer Institute in Chicago, and who died of lymphoma. No one could complain that Dr. Raza criticizes oncology in the abstract. I found myself in agreement with Azra throughout most of the book. We both share a similar philosophy about medicine and are critical that its modern application focuses too much on diseases and cures (of which it is largely impotent), rather than illness and healing.
Dr. Raza rightfully pushes back against the incessant hype of “game changers” and “block buster” therapies promised by the pharmaceutical industry and propagated in the media and academic press. The blunt fact is that the vast majority of cancer patients receive either surgery (slash), chemotherapy (poison), and/or radiation therapy (burn). The dream of personalized and targeted oncology is just that: a dream. Though the slash-poison-burn regime for cancer patients is brutal, Dr. Raza is clear that untreated cancer is even more destructive. The book does a good job at not providing cover for homoeopathic kooks. The contemporary hype cycle of cancer research has now become an insult to the vast majority of patients who will see nothing of these “break-throughs” so often mentioned.
The treatment landscape for AML has not evolved much in fifty years, nor has it for most of the common types of cancers. With minor variations, a protocol of surgery, chemotherapy, and radiation–the slash-poison-burn approach to treating cancer–remains unchanged. It is an embarrassment. Equally embarrassing is the arrogant denial of that embarrassment. Technologic advances and the cure of cancer in animal models are loudly proclaimed as if those successes have had anything to do with treating the disease in humans. Improvement in survival of cancer patients measured in weeks is regularly refereed to as a “game changer.” To make rosy pronouncements is profoundly unfair to patients. No one is winning the war on cancer. It is mostly hype, the same rhetoric from the same self-important voices for the past half a century.
Critics of foreign aid often point out that despite a trillion dollars of cash being transferred from the developed to the developing world, the countries that have received the most aid dollars remain the poorest ones today. Wealthy countries have collectively spent hundreds of billions of dollars of money into basic cancer research since the 1970s. If the long-term goal of this research was to extend the lives of most cancer patients by years, then we have seen a remarkably poor return on investment. Dr. Raza’s core thesis is that the structure and incentives of cancer research and development has led to this failure.
Why can’t we make use of the millions of research papers published in the pasty fifty years claiming huge successes in understanding the biology of cancer? For four decades, I have been hearing the same glowing predictions about the magic treatment just around the corner, resulting from a better understanding of oncogenes, tumor suppressor genes, the human genome and transciptome, the immune system, or choking off blood supply to tumors. Most have fallen flat when brought to the beside. The gaping disconnect between knowledge about cancer biology and the capacity to use this knowledge to benefit patients is staggering.
Headlines about cancer treatments are almost always the few success stories that work for a small subset of patients. This has warped the public’s perception of the progress of cancer research. It is no surprise that cancer patients are desperate to get access to “experimental” therapies, even though the vast majority of these treatments fail to improve survival outcomes over existing standards of care. Two early “magic bullet” successes of cancer treatment have played an outsize role in influencing the expectations of cancer therapeutics. Acute promyelocytic leukemia (APL) used to be a death sentence but now has a 10-year survival rate of 90% thanks a treatment that is based on a derivative of vitamin A. For patients with chronic myeloid leukemia (CML), the tyrosine kinase inhibitor imatinib has been able to double 5 year survival rates to around 90%. These treatments for both APL and CML are remarkably effective because the genetic mechanism of these cancers is surprisingly simple. In the case of CML, the cancer is caused by a fusion of the BCR-ABL genes. Treatments which block transcripts from this gene effectively shut down the cancer.
The success in treating CML and APL patients belies the maddening biological complexity of most cancers. As Raza points out “[t]reating cancer as one disease is like treating Africa as one country”. The hype around antiangiogenesis treatments has moved almost seamlessly to claims about immunotherapies being a “game changer”, “breakthrough”, and a “miracle in the making”.[1] Even if there aren’t any imatinibs or ATRA-ATOs around the corner, is the problem really that bad? In short: yes. Over the years 2005-2016 the probability that a cancer drug would succeed in passing through all four stages of drug development was a dismal 5%. This number is bad in both absolute and relative terms, with oncology having the lowest probability of success among 14 major disease areas. In other words, 95% of experimental cancer drugs fail at either Phase I, II, III, or during regulatory filing. The greatest chance of failure occurs from phase II to III (which means that no biologic effect can be demonstrated). And even when a cancer drug is amongst the 5% that get approved, the median increase in survival is a paltry 2.7 months.[2]
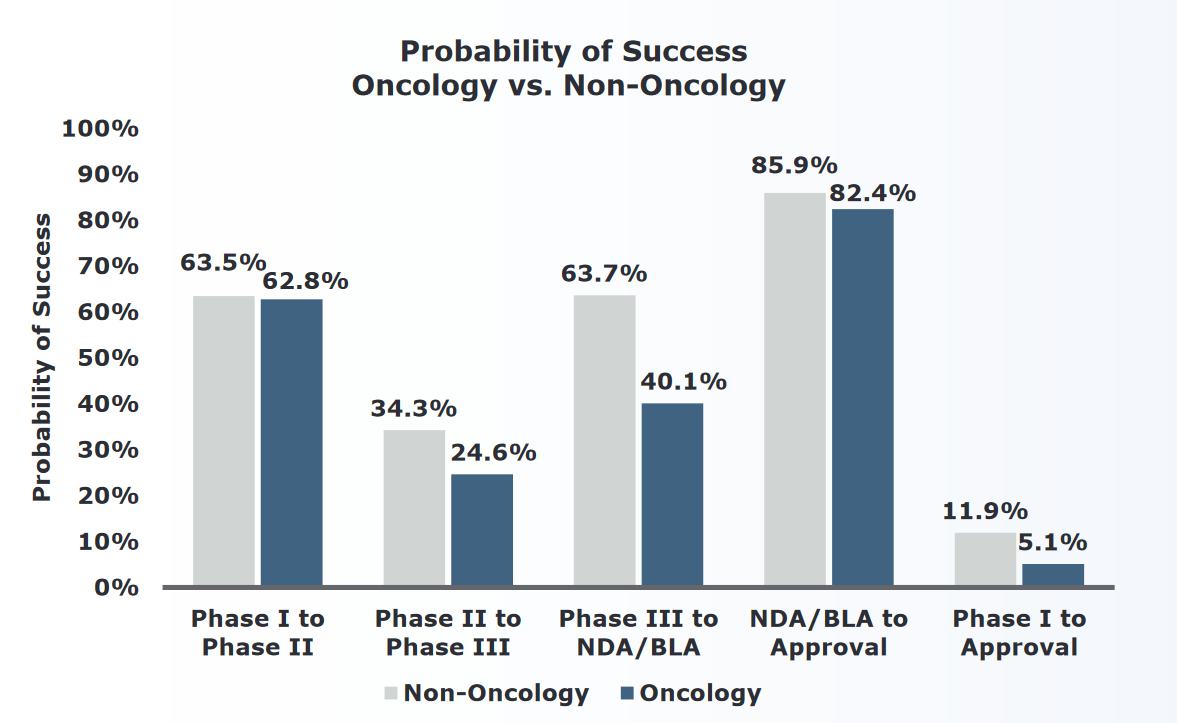
Oncology approval rates
The First Cell attempts to explain why therapeutic translation in oncology has been so bad. The book suggests that bad incentives affect researchers and pharmaceutical companies alike. Azra notes that most treatment “innovations” involve developing biosimilar drugs that are based off some targeting strategy that has been previously approved. Because drug pricing is wholly unconstrained in the United States, drug companies can earn billions of dollars of profits from merely getting their drug across the approval line. The case of paclitaxel (Taxol) is illustrative. Cancer researchers assumed that Taxol worked by inhibiting mitosis, and 25 cancer drugs were developed based on this theoretical mechanism. Amazingly, all 25 of them failed.
Recent success stories like CAR-T therapy for paediatric acute lymphoblastic leukemia (ALL), have generated “sensationalized” media reports that will encourage the development of “voguish” therapies, according to Raza. Furthermore, these celebratory stories about immunotherapy treatments like CAR-T often fail to mention their fatal side effects. Because genetically engineered T cells are so effective at killing leukemia cells expressing the CD19 antigen, they can quickly overwhelm the kidneys when the cells break apart (lysis). Unless treated rapidly, tumour lysis syndrome and cytokine storms can lead to organ failure and death.
The first fatal adverse event due to off-tumour recognition by a CAR occurred in a patient with colorectal cancer treated with high numbers of T cells expressing a third generation of CAR targeting eRBB2/HER2. The patient developed respiratory distress and cardiac arrests shortly after the T cell transfer and died of multisystem organ failure 5 days later.
Raza takes aim at cancer researchers for continuing to use a flawed system of biological experiments that relies of cell lines and animal models: “..using even more artificial systems of genetically engineered animals are as realistic as dissecting the brain and expecting to discover consciousness”. The current paradigm of drug development for cancer goes something like this. A compound or molecular target is discovered through basic research. The promising target “works” well in killing cancer cells preserved in cell line (i.e. petri dishes). Then, a mouse model is either genetically modified or xenograted with cancer cells and develops the cancer of interest. The target “works” in the mouse model.
Treating cell lines and mouse models is a poor proxy for human disease for multiple reasons. The cell lines that are used for in silico testing have a transciptomic landscape that meaningfully contrasts a normal cancer cell. The reason is simple: the expression profile a cancer cell needs to survive in a petri dish is radically different from the one that is needed to proliferate in a human tissue. As a result, cancer cell lines are often more genetically similar to cell lines from different organs then they are to their respective organs in situ! Even though humans and mice last shared a common ancestor 80 million years ago, we have remarkably similar genes and predispositions to cancer. However the way cancer develops and its responsiveness to different treatments has been shown, time and time again, to be different between the two species. Furthermore, “giving” a mouse model cancer often involves destroying its immune system so the foreign cells won’t be rejected. The odds of an effective treatment on an immunocomprised mouse translating to humans is, unsurprisingly, limited.
As a result, all the accompanying reactions of the body, the counterpunch resulting in a misdirected immune response, the system-wide reaction to the presence of malignancy, the whole array of paraneoplastic syndromes are entirely absent in animal models. Who has catalogued the B-symptoms of joint pains and night sweats in animals?
Raza’s critique of the cancer research and drug development paradigm is convincing. Some of her suggested solutions also seem reasonable: “[p]revention will be the only compassionate, universally applicable cure.” If cancer is a wicked problem, then the best solution would be not to have the problem to begin with. Human nature will always prefer the idea of a cure over prevention. When one considers that there are some species that almost never develop cancer (Peto’s Paradox), the hope of a cure seems all the more reasonable. For example, elephants and whales, which have a thousand-fold more cells than humans, largely live cancer-free lives. For elephants, this may be because they have twenty copies of the p53 tumour suppressor gene! Unfortunately having this many copies of a gene is not a feasible strategy for our species.
As the human body ages it becomes more susceptible to cancer. Raza summarizes these vulnerabilities into the “MIST” of ageing: mutations, immune system deterioration, senescent cells, and tissue loss. Furthermore, when cancer does develop in an older person, it will almost certainly be more genetically complex due to the accumulation of mutations. In contrast, childhood cancers are usually the result of a single malfunctioning gene. Because the clonal landscape of childhood cancers is less complex, targeted therapies are more likely to work. With less genetic variation, cancer has fewer opportunities to leverage natural selection. The MIST factors further point the benefit of prevention over treatment, as the latter will be less effective.
Unfortunately Raza’s idea of cancer prevention goes beyond the usual epidemiological risk factors. Instead, she endorses a wholly unproven approach that would, based on her own critiques, be ripe for failure: liquid biopsies and leveraging “big data” for early detection. For example, the Harvey chapter is ebullient about cell-free DNA (cf-DNA) and circulating tumour DNA (ct-DNA) biomarkers. At times I was almost embarrassed by the quantity of buzzwords she used in the chapter (MicroRNAs, lab on a chip, and proteomics, to name a few). I would have a hard time keeping a straight face writing this:
The era of big data, cloud computing, artificial intelligence, and wearable sensors has arrived. The study of cancer is evolving into a data-driven, quantitative science. Merging information obtained from liquid biopsies (RNA, DNA, proteomics, exosome studies, CTC), with histopathology, radiologic, and scanning techniques, aided by rapid machine learning, image reconstruction, intelligent software, and microfluidics can, and will, revolutionize the way we diagnose and prevent rather than treat cancer in the future. The ideal strategy will emerge from harnessing cutting-edge technology for a multidisciplinary systems biology approach through a consilience of scientists with expertise in molecular genetics, imaging, chemistry, physics, engineering, mathematics, and computer science.
This paragraph feels like a researcher applying for a grant, rather than a sober critic of cancer research. Ironically Raza is aware that “…population-based, conventional screening programs costing astronomical sums of money have not yielded the dramatic success that was expected… [and] can result in overdiagnosis and overtreatment.” She also acknowledges that “detecting” cancer early is less powerful that common sense might suggest. Detected cancers can be thought of as birds, tortoises, and hares. Birds are the most aggressive cancers that cannot be caught even after they are found. Hares are also aggressive cancers, but can be slowed down or stopped. Lastly tortoises are those cancers that would grow sufficiently slowly that a patient will likely die of natural causes before the cancer takes over. Screening is therefore only helpful for hares.
Using machine learning tools to predict pre-symptomatic cancer is a recipe for disaster. In 2016 the US Preventive Services Task Force made a recommendation that women receive biannual mammographies rather than annual ones. The reason? Most breast cancer “findings” amounted to false positives which impose a psychological, and possibly physical, harm on women. The idea of a “rapid” machine learning tool sending cancer warnings would overwhelm any screening system with false alerts. The value of pre-symptomatic screening is proportional to the underlying detection signal and disease prevalence. For example, in the case of Li-Faumeni syndrome, a strong argument could be made for frequent testing since most of these patients will develop cancer before the age of 30. For society as a whole, early detection of cancer is infeasible because the prevalence of cancer is too low and the signals found in liquid biopsies are too uncertain.
One other criticism I have of the book is that the references are poorly structured and sometimes misleading. Furthermore, there are no numbered sources, so the reader must infer which sources match which statements in the book. At one point Raza states that “… p53 mice aged rapidly, and within months, they looked very old, and their lifespans shrank by 30 percent.” However the reference she provides actually shows the opposite result, and Raza is likely referring to early studies when genetic engineering tools were less effective. Or consider the statement: “[t]hose few drugs that are approved might as well have failed; once they are administered in non-trial settings, the results are no better than those that were not approved.” I was intrigued by this claim but could not determine its source from the appendix.
I have my disagreements with The First Cell, but I am glad it was published. The book provides a necessary reality check for industry cheerleaders and sensational headlines emanating from the media. Dr. Raza is a deeply empathetic person and she talks about her patients with such sensitivity that they almost come alive to the reader. Poetry readers will also be delighted that the book is sprinkled with English and Urdu poems (some of which the author translated). Where The First Cell falls short, it does so with good intentions.
Notes
-
One particularly galling example of media hype about an antiangiogenesis treatment that was effective in mice was a NYT article from 1998 in which the Nobel prize winner James D. Watson said that “Judah [Folkman] is going to cure cancer in two years.” Oncologists around the country were inundated with calls from their patients the next day demanding access to this magical treatment. ↩
-
This number is surely an overestimate as it includes increases in progression free survival (PFS), which is, at best, a noisy proxy for overall survival. Furthermore there is a statistical filter for approved drugs (remember 95% fail), so even if they have an effect, the mean effect is likely much smaller. ↩
